Publications
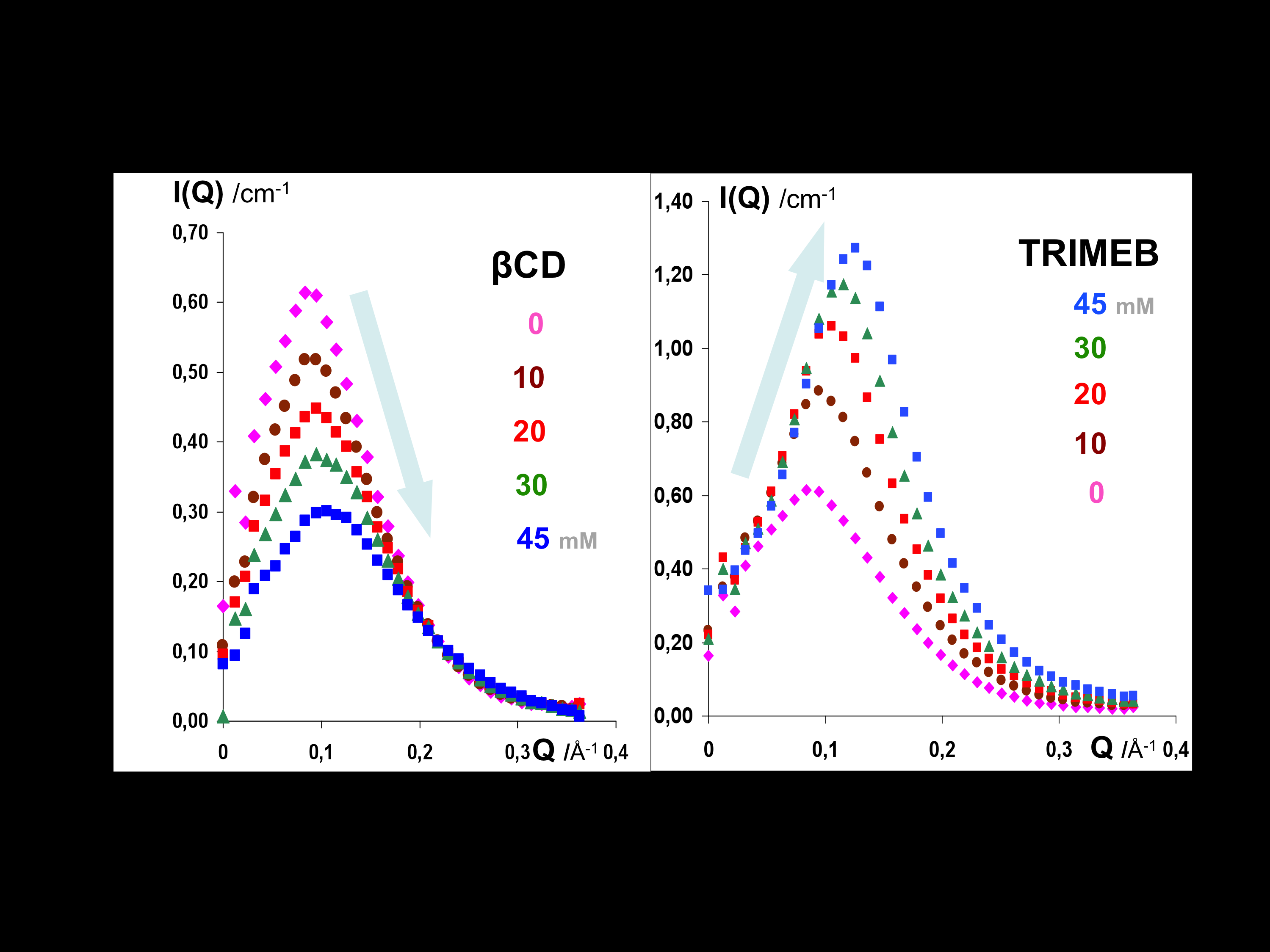
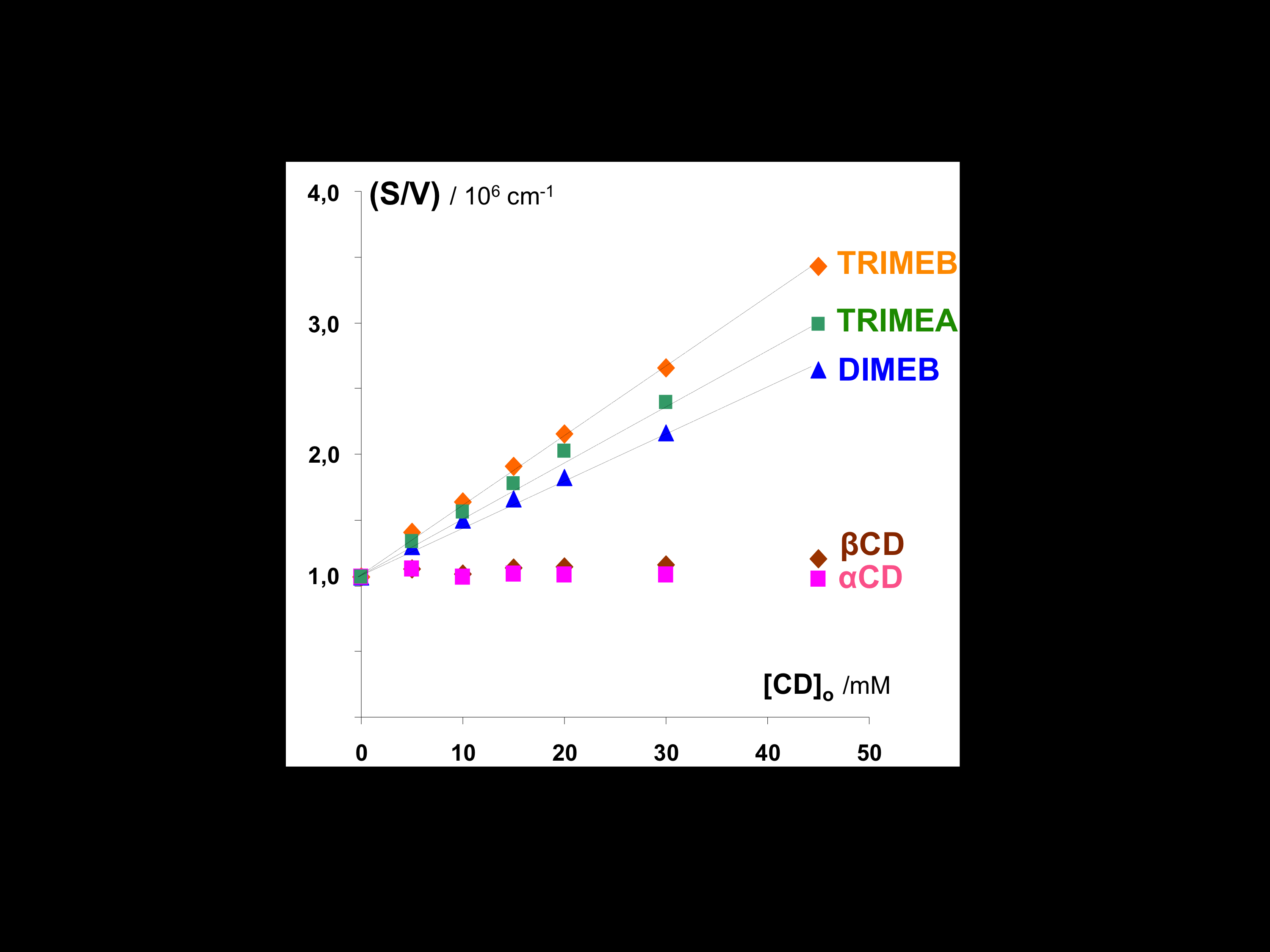
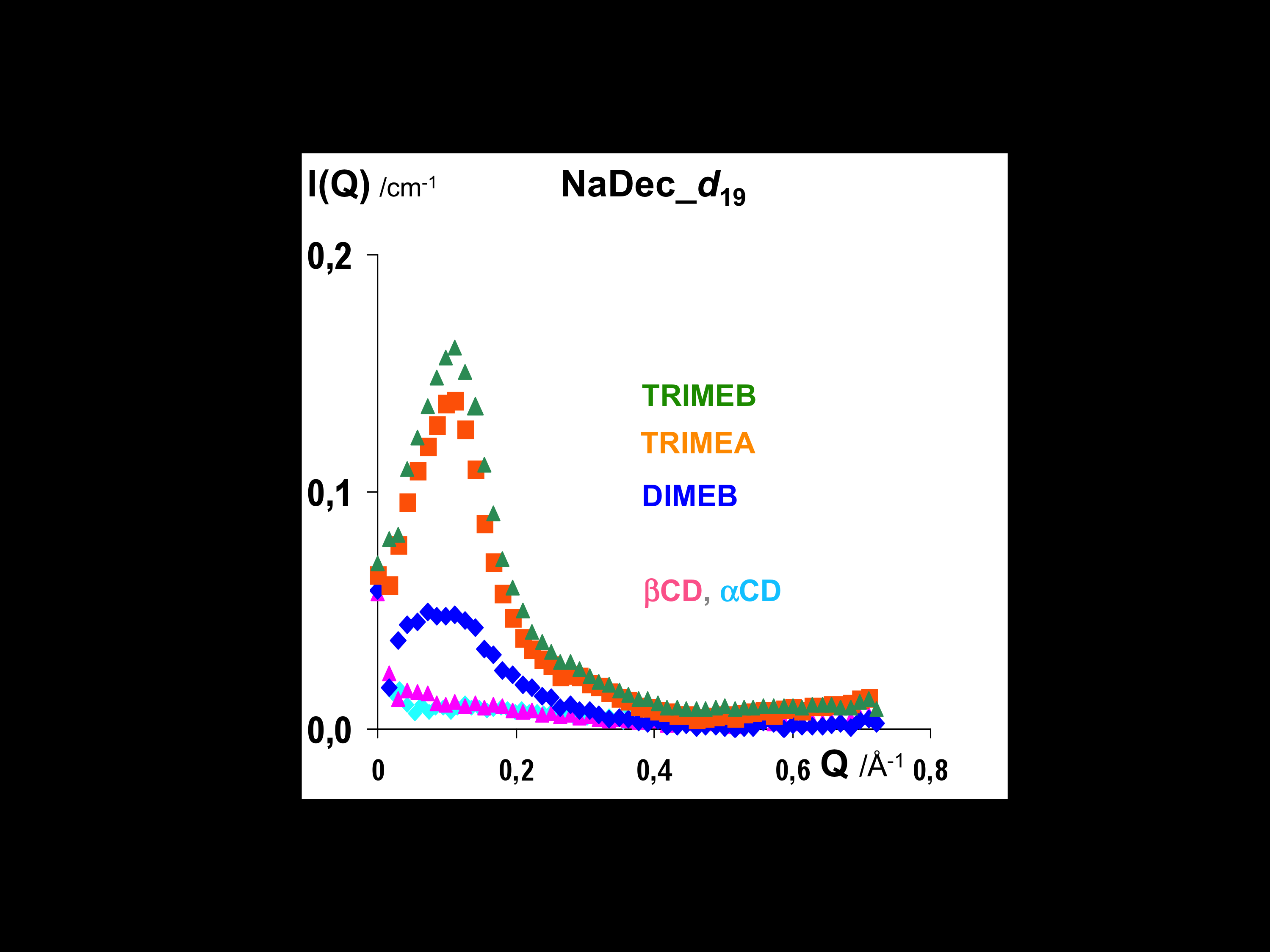
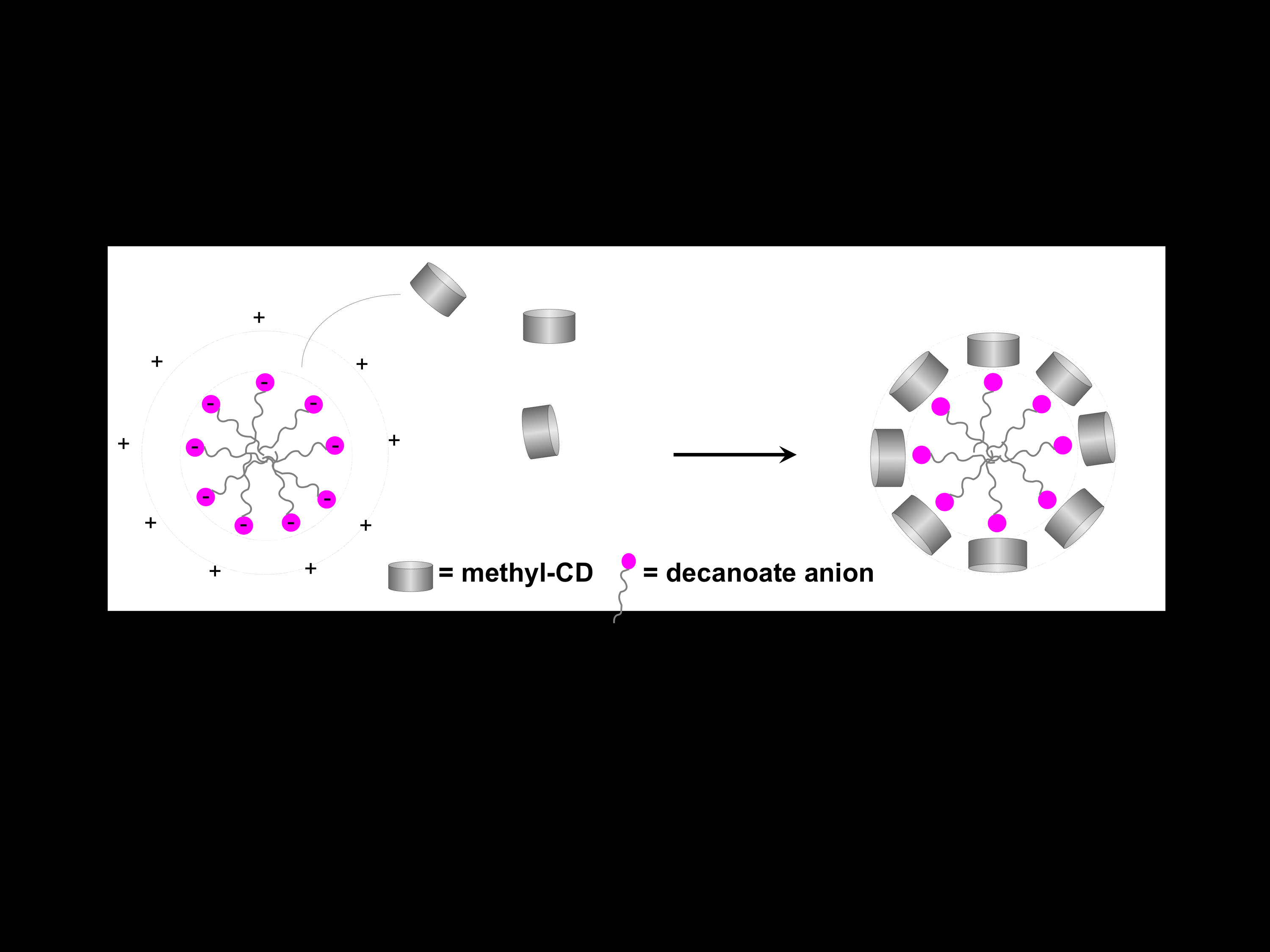
Why Do Methylated and Unsubstituted Cyclodextrins Interact So Differently with Sodium Decanoate Micelles in Water?
Andrade-Dias, C; Lima, S; Teixeira-Dias, JJC; Teixeira, J
2008, JOURNAL OF PHYSICAL CHEMISTRY B, 112, 48, 15327-15332.
How does beta-cyclodextrin affect the aggregation of sodium perfluoroheptanoate in aqueous solution: a F-19 NMR study
Lima, S; Andrade-Dias, C; Dias, AMA; Marrucho, IM; Coutinho, JAP; Teixeira-Dias, JJC
2007, JOURNAL OF INCLUSION PHENOMENA AND MACROCYCLIC CHEMISTRY, 57, 1-4, 157-162.
Inclusion complexes of 2-phenoxyethanol and alkoxyethanols in cyclodextrins: an H-1 NMR study
Andrade-Dias, C; Goodfellow, BJ; Cunha-Silva, L; Teixeira-Dias, JJC
2007, JOURNAL OF INCLUSION PHENOMENA AND MACROCYCLIC CHEMISTRY, 57, 1-4, 151-156.
From simple amphiphilic to surfactant behavior: Analysis of H-1 NMR chemical shift variations
Andrade-Dias, C; Lima, S; Teixeira-Dias, JJ
2007, JOURNAL OF COLLOID AND INTERFACE SCIENCE, 316, 1, 31-36.
Competition between the hydrated fluoride anion and hexanoic acid for inclusion in beta-cyclodextrin: An H-1-NMR study in aqueous solution
Lima, S; Goodfellow, BJ; Teixeira-dias, JJC
2006, JOURNAL OF INCLUSION PHENOMENA AND MACROCYCLIC CHEMISTRY, 54, 1-2, 35-40.
How does beta-cyclodextrin affect oxygen solubility in aqueous solutions of sodium perfluoroheptanoate?
Dias, AMA; Andrade-Dias, C; Lima, S; Coutinho, JAP; Teixeira-Dias, JJC; Marrucho, IM
2006, JOURNAL OF COLLOID AND INTERFACE SCIENCE, 303, 2, 552-556.
Controlled synthesis of morphological well-defined BiVO4 pigment particles supported on glass substrates
Cruz, SG; Girginova, PI; Neves, MC; Smolka, P; Kusak, R; Caldeira, M; Teixeira-Dias, J; Pedrosa, J; Trindade, T
2006, ADVANCED MATERIALS FORUM III, PTS 1 AND 2, 514-516, 1211-1215.
Inclusion of molybdenocene dichloride (Cp2MoCl2) in 2-hydroxypropyl- and trimethyl-beta-cyclodextrin: Structural and biological properties
Braga, SS; Marques, MPM; Sousa, JB; Pillinger, M; Teixeira-Dias, JJC; Goncalves, IS
2005, JOURNAL OF ORGANOMETALLIC CHEMISTRY, 690, 12, 2905-2912.
Inclusion complex formation of diferrocenyldimethylsilane with beta-cyclodextrin
Fernandes, JA; Lima, S; Braga, SS; Ribeiro-Claro, P; Rodriguez-Borges, JE; Teixeira, C; Pillinger, M; Teixeira-Dias, JJC; Goncalves, IS
2005, JOURNAL OF ORGANOMETALLIC CHEMISTRY, 690, 21-22, 4801-4808.
Solid-state inclusion compounds of small amphiphilic molecules (CnEm) in beta-cyclodextrin: a study at defined relative humidities
Cunha-Silva, L; Teixeira-Dias, JJC
2005, NEW JOURNAL OF CHEMISTRY, 29, 10, 1335-1341.
Classroom questioning: teaching strategies to enhance learning

Teaching strategies
Albergaria-Almeida P, Teixeira-Dias JJC
2012, New York, Nova Science Publishers.
Implementing a constructivist learning environment: students’ perceptions and approaches to learning

Beyond Transmission - Innovations in University Teaching
Albergaria-Almeida P, Teixeira-Dias JJC
2011, Oxfordshire, Libri Publishing.
How can critical thinking be recognised in the classroom?

Critical Thinking
Albergaria-Almeida P, Teixeira-Dias JJC
2011, New York, Nova Science Publishers.
ISBN:
978-1-61324-419-7
Teaching and Learning Chemistry: a new approach at the University of Aveiro, in Portugal

In N. Popov, C. Wolhuter, B. Leutwyler, M. Mihova & J. Ogunleye (Eds.), Comparative Education, Teacher Training, Education Policy, School Leadership and Social Inclusion
Almeida, P., Teixeira-Dias, J.J., & Martinho, M.
2010, 357-362, Sofia, Bureau for Educational Services.
ISBN:
978-954-9842-15-9
Building a culture of Creativity while engaging science students in inquiry

In Claus Nygaard, Nigel Courtney, Clive Holtham (Eds.), Teaching Creativity - Creativity in Teaching.
Almeida P, Teixeira-Dias JJ, Medina J
2010, 85-101, Oxfordshire, Libri Publishing.
ISBN:
978-1907471179
Nanostructured Metals In Raman Spectroscopy

In Nalwa HS (Eds.), Encyclopedia Of Nanoscience And Nanotechnology
Nogueira HIS, Teixeira-Dias JJ, Trindade T
2004, 7, 699-715, California, American Scientific Publishers.
ISBN:
1-58883-001-2