Nature Reviews Chemistry has just highlighted, in the Research Highlights section, the work carried out within the scope of Dinis Abranches Master's thesis in Chemical Engineering, developed in a research group of CICECO-Aveiro Institute of Materials, in partnership with York University. The work also counted with the collaboration of researchers from the University of Zaragoza and the Polytechnic Institute of Bragança.
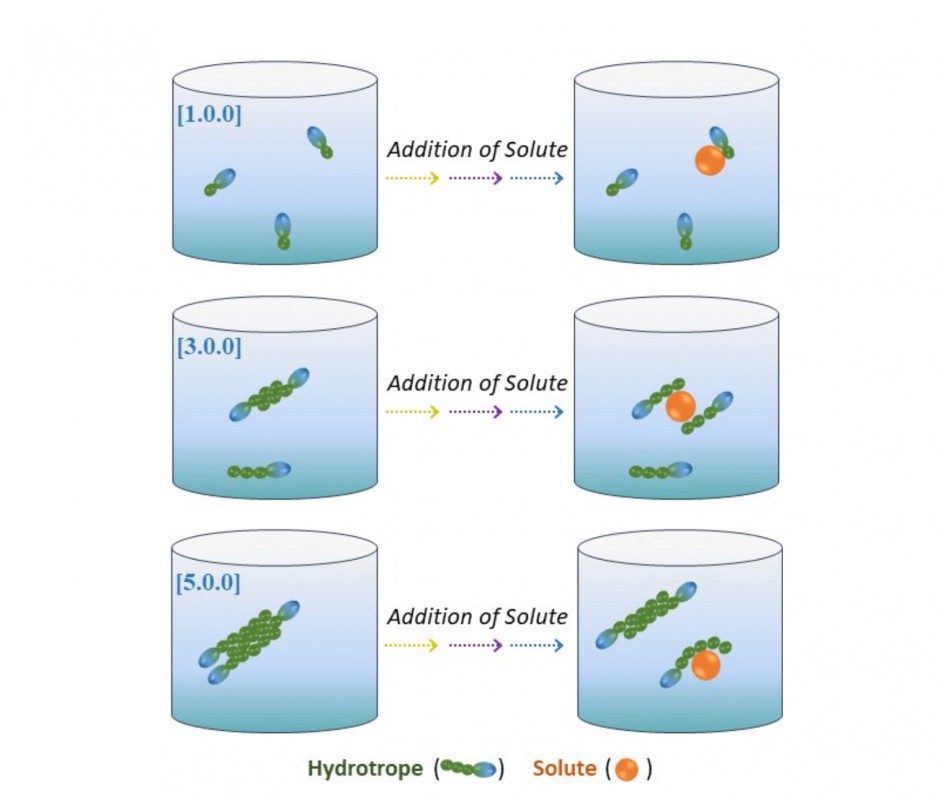
Using proton Nuclear Magnetic Resonance (NMR), the article provides, for the first time, experimental evidence for a hydrotropic mechanism, i.e., the increase of solubility of a hydrophobic (insoluble) compound in the presence of a third component (hydrotrope), a mechanism recently proposed and based on statistical thermodynamics. In addition, through computational techniques, the article reports a new way of quantifying the apolarity of a molecule, which is used to probe the influence of apolarity on the behavior of hydrotropy.
Changes the mental picture about hydrotropy
 Experimental work in this phase of fundamental science (in chemistry) involved phenolic compounds, insoluble in water, and glycerol ethers used as hydrotropes; according to Dinis Abranches, it makes it possible to imagine diverse applications that are not part of the objectives of the current study, but that may involve the increase of solubility of compounds in many other situations, such as drugs or hydrocarbons. These ethers are not toxic, are biodegradable, and are produced from glycerol, which is a green, cheap raw material and a byproduct of biodiesel production, he says.
Experimental work in this phase of fundamental science (in chemistry) involved phenolic compounds, insoluble in water, and glycerol ethers used as hydrotropes; according to Dinis Abranches, it makes it possible to imagine diverse applications that are not part of the objectives of the current study, but that may involve the increase of solubility of compounds in many other situations, such as drugs or hydrocarbons. These ethers are not toxic, are biodegradable, and are produced from glycerol, which is a green, cheap raw material and a byproduct of biodiesel production, he says.
It is now necessary, he adds, to do application-oriented research: identify situations where hydrotropy can be useful and, making use of the theoretical knowledge developed, design the ideal hydrotropes for specific situations.
Hydrotropy is therefore a highly relevant topic in the development of sustainable processes and products, team members argue. In the text published in Nature Reviews Chemistry, the supervisor of the work, João Coutinho, professor of the Department of Chemistry of the UA, considers that hydrotropy has been “neglected in the debate on the future of green chemistry”, environmentally friendly. “Probably due to insufficient knowledge of its molecular mechanisms,” he suggests.
Thus, the work developed not only clarifies the molecular mechanism of this phenomenon, but also explores its ramifications, leading to the rationalization of the development of new hydrotropes. João Coutinho highlights the possible contribution of this study to the construction of a new mental framework on hydrotropy.
Featured in Nature Reviews Chemistry:
Graziano, G.
Solving a solubility problem.
Nat Rev Chem (2020).
https://doi.org/10.1038/s41570-020-0202-3
Featured article:
Dinis O Abranches, Jordana Benfica, Bruna P Soares, Alejandro Leal-Duaso, Tânia E Sintra, Elísabet Pires, Simão P Pinho, Seishi Shimizu, João A P Coutinho.
Unveiling the mechanism of hydrotropy: evidence for water-mediated aggregation of hydrotropes around the solute.
Chem. Commun (2020).
Related Articles
We use cookies for marketing activities and to offer you a better experience. By clicking “Accept Cookies” you agree with our cookie policy. Read about how we use cookies by clicking "Privacy and Cookie Policy".















