With the current technology and consumer market trends, we are in a great demand for portable energy storage, which is satisfied now and will be satisfied in the near future by electrochemical rechargeable batteries. In 2011 MXene family of materials was discovered in the USA, which showed a great potential to store much more energy in the same volume and to accumulate and release the energy much faster than the materials currently used in the batteries.
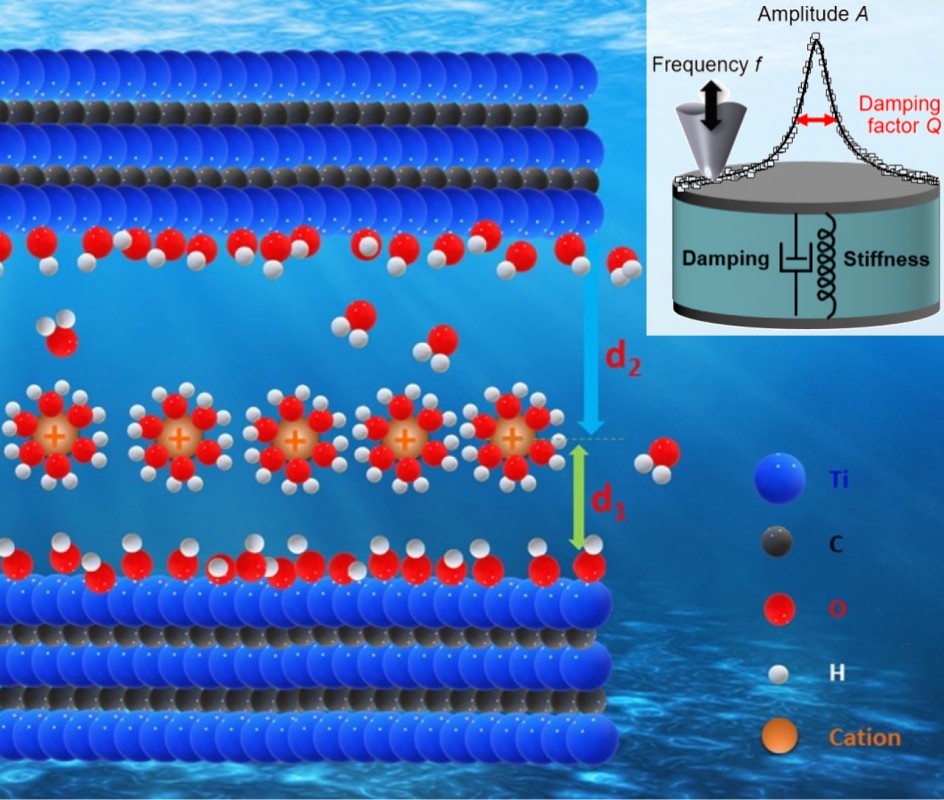
At the atomic scale, MXenes consist of multiple thin layers separated by tight gaps, which are places accommodating ions (the working particles carrying the energy transformations in batteries) and releasing them on demand. Such MXene’s unique structure allows storing a significantly larger number of ions in the same volume compared to the materials used in conventional batteries but the exact mechanisms are elusive with multiple unknowns about the material “life” at the atomic scale.
In this paper, the team of co-authors explored how different ions enter the MXene structure and reside there and how the electrolyte water, carrying the ions, adapts itself to the tight space between MXene layers. The team of researchers lead by Oak Ridge National Laboratory in the USA with participation of Drexel University, Tulane University, Georgia State University in the USA, Southeast University in China, and the University of Aveiro in Portugal looked simultaneously at multiple different aspects of the processes in the MXene with the help of a set of sensitive experimental methods and atomic scale computer simulations. The work provides key insights and advances understanding of the MXene’s striking capabilities opening the door to engineering MXenes and devices utilizing them.
“The is a great example of a very successful cooperation of scientist separately developing different novel experimental and numerical techniques, which are then brought together and focused on one problem,” says Alexander Tselev, a researcher of the Department of Physics and CICECO at the University of Aveiro and a co-author of the paper, who contributed to the development of a microscopy method that allows visualization of the ion distribution in the subsurface layers of MXene with a very high spatial resolution of the about 10 nm (1 nm is one millionth part of a millimeter) directly in liquid electrolytes, an important capability unavailable before.
The paper was published in the top 1% journal (JCR 2019) Energy & Environmental Science this month:
Q. Gao, W. Sun, P. Ilani-Kashkouli, A. Tselev, P. Kent, N. Kabengi, M. Naguib, M. Alhabeb, W.Y. Tsai, A. P. Baddorf, J. Huang, S. Jesse, Y. Gogotsi, and N. Balke, Tracking ion intercalation into layered Ti3C2 MXene films across length scales, Energy & Environmental Science (2020). DOI: 10.1039/D0EE01580F
Related Articles
We use cookies for marketing activities and to offer you a better experience. By clicking “Accept Cookies” you agree with our cookie policy. Read about how we use cookies by clicking "Privacy and Cookie Policy".











