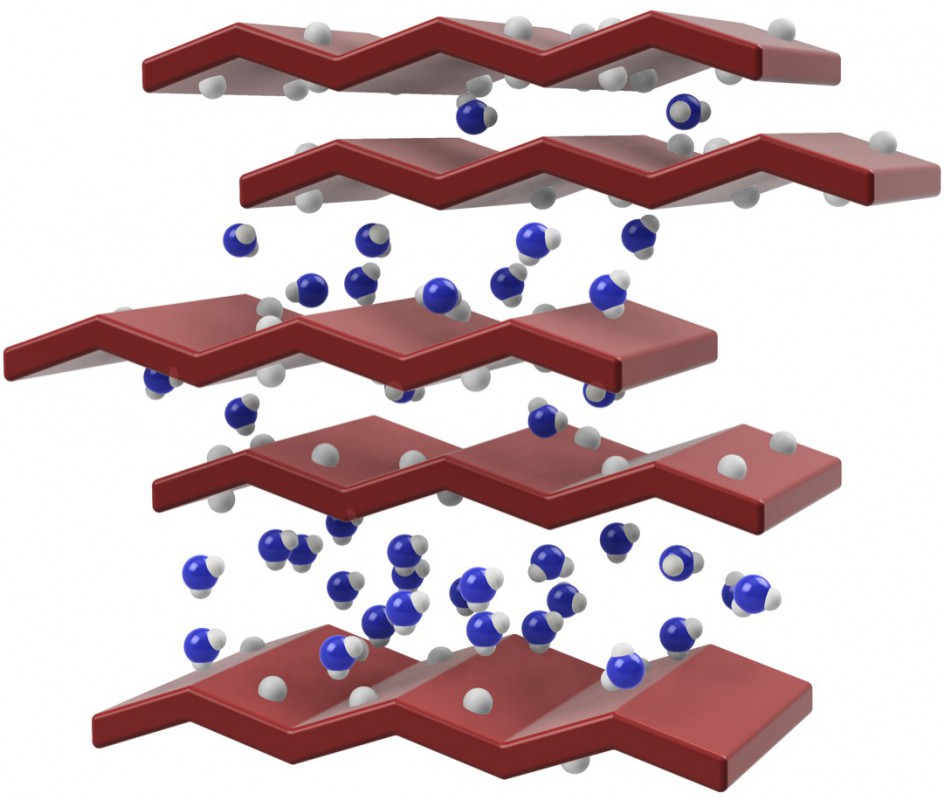
Researchers from CICECO – Aveiro Institute of Materials, belonging to the Departments of Chemistry (Filipe A. Almeida Paz, Ricardo F. Mendes and Patrícia Silva) and Materials & Ceramic Engineering (Filipe Figueiredo, Paula Barbosa and Eddy Domingues), discovered that the in situ conversionof the layered [Gd(H4nmp)(H2O)2]Cl∙2H2O into [Gd2(H3nmp)2]∙xH2O at high humidity and temperature (x = 1 to 4 water molecules; H6nmp stands for nitrilotris(methylenephosphonic) acid), led to a remarkable increase in proton conductivity. The scientists discovered that the key factor for this high proton conduction is the unusual dynamic structural transformation with the insertion of water molecules with the concomitant outlet of chloride ions.
Two decades of research on Metal-Organic Frameworks
The research group of Filipe A. Almeida Paz has been working for several years on the synthesis, characterization and application of lanthanide-based polyphosphonate materials. Many of these compounds have shown to be excellent heterogeneous catalysts over the years, embodying also other properties such as photoluminescence.
“The structure of the newly reported material, [Gd2(H3nmp)2]∙xH2O, eluded us for many years” stressed Filipe A. Almeida Paz. “In fact, it was first prepared over 13 years ago by my first post-doctoral student, Luís Cunha-Silva, now an independent researcher at the University of Porto. The powder X-ray diffraction pattern showed a “poorly” crystalline material which was discarded for several years. My first Ph.D. student, Patrícia Silva (now working in the U.K. at the company Dyson), obtained this phase several times, but we never managed to solve the structure. Only when Ricardo Mendes came to work in Aveiro that we managed to grow a poor single-crystal which unveiled part of the crystalline structure of the material. The big surprise was when we observed in situ the transformation of [Gd(H4nmp)(H2O)2]Cl∙2H2O, a fantastic catalyst whose structure was published a few years ago in Chemistry-A European Journal, into something whose powder pattern resembled this scattered information over the years.”
Remarkable Conductivity
The samples were passed to Figueiredo’s group for detailed studies of the dynamic water vapour sorption and of the electrical properties under variable temperature and humidity. Eddy Domingues and Paula Barbosa carried out those measurements that could monitor in situ the phase transformation of the parent compound into one of the best proton-conducting Metal-Organic Framework with conductivity of 0.51 S cm-1 at 94 °C and 98% relative humidity. This value represents an increase of about five orders of magnitude with respect to the parent compound. The compound can also be directly obtained by several synthetic approaches, showing conductivity values in the range of 3.79×10-2 S cm-1 in the same conditions. The key factor for high proton conduction is the unusual dynamic structural transformation with water insertion and release of chloride ions.
“This work can be seen as a “proof of concept” to better design and prepare new MOF-type materials based on simple “anionic exchange” processes” adds Filipe A. Almeida Paz. “After these astounding results we are now embarking on an active research path which is focused on this type of chemical transformations to see if we can further improve the properties of many of our MOFs.”
Article Reference
Enhanced Proton Conductivity in a Layered Coordination Polymer
Ricardo F. Mendes, Paula Barbosa, Eddy M. Domingues, Patrícia Silva, Filipe Figueiredo, Filipe A. Almeida Paz
Chemical Science, 2020, Volume 11, Pages 6305-6311
DOI: 10.1039/d0sc01762k
https://pubs.rsc.org/en/content/articlelanding/2020/SC/D0SC01762K
Related Articles
We use cookies for marketing activities and to offer you a better experience. By clicking “Accept Cookies” you agree with our cookie policy. Read about how we use cookies by clicking "Privacy and Cookie Policy".














