
Catalysis science is crucial for the sustainable development of a technologically advanced society. An important goal in transition metal catalysis is the development of systems that combine the advantages of homogeneous catalysts (such as, for example, high activities and selectivities) with those of heterogeneous catalysts (such as ease of catalyst-product separation and catalyst recycling). To date, one of the main strategies to accomplish this goal has been the immobilization of molecular catalysts onto solid supports. The resultant materials suffer from numerous drawbacks which include catalyst “leaching”, leading to deactivation, and poor accessibility of the active sites. A very attractive alternative approach is to use a catalyst that is fully soluble in the reaction mixture containing the reagents but which precipitates out of solution once the reagents have been consumed, allowing it to be separated from the products without using additional chemical solvents. Despite the great appeal of self-separating catalysts, finding ones that work correctly is extremely difficult.
Now, researchers from CICECO and the Department of Chemistry at the University of Aveiro have discovered a new type of self-precipitating catalyst for oxidation reactions that use aqueous hydrogen peroxide (H2O2) as an environmentally friendly oxidant. The catalyst precursor is the molybdenum oxide-triazole hybrid material [MoO3(trz)0.5] (1), which has a two-dimensional (2D) solid-state structure consisting of metal oxide layers and metal-coordinated 1,2,4-triazole (trz) ligands. When applied as a catalyst for the oxidation of alcohol, aldehyde, olefin and sulfide substrates, compound 1 forms a soluble active species by the action of H2O2. Exhaustion of the oxidant leads to spontaneous reassembly of the 2D structure of 1 and the compound precipitates, allowing straightforward recovery and reuse. The hybrid 1 is the first example of a self-precipitating catalyst that has an extended 1D, 2D or 3D crystalline structure. Interestingly, the tungsten analogue of 1, [W2O6(trz)], does not exhibit this behaviour, but can instead be used as a conventional heterogeneous catalyst.
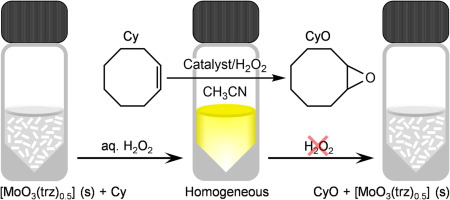
Figure. Schematic representation of the epoxidation of cis-cyclooctene (Cy) with 1, using H2O2 as oxidant. Compound 1 reacts with the oxidant to give a homogeneous solution (active species) and the epoxidation reaction is catalyzed. Upon completion of epoxidation and exhaustion of the oxidant, 1 spontaneously precipitates.
 The work was published in the August issue (2016) of the Journal of Catalysis and was selected by the Editor-in-Chief as one of four Featured Articles. The Journal of Catalysis is the premier scholarly publication in the field of catalysis. Featured Articles are selected due to the quality of the research, the clarity of the exposition or the novelty of the findings, and are made freely available to the public for 3 months following publication of the respective issue.
The work was published in the August issue (2016) of the Journal of Catalysis and was selected by the Editor-in-Chief as one of four Featured Articles. The Journal of Catalysis is the premier scholarly publication in the field of catalysis. Featured Articles are selected due to the quality of the research, the clarity of the exposition or the novelty of the findings, and are made freely available to the public for 3 months following publication of the respective issue.
Related Articles
We use cookies for marketing activities and to offer you a better experience. By clicking “Accept Cookies” you agree with our cookie policy. Read about how we use cookies by clicking "Privacy and Cookie Policy".
















