Abstract
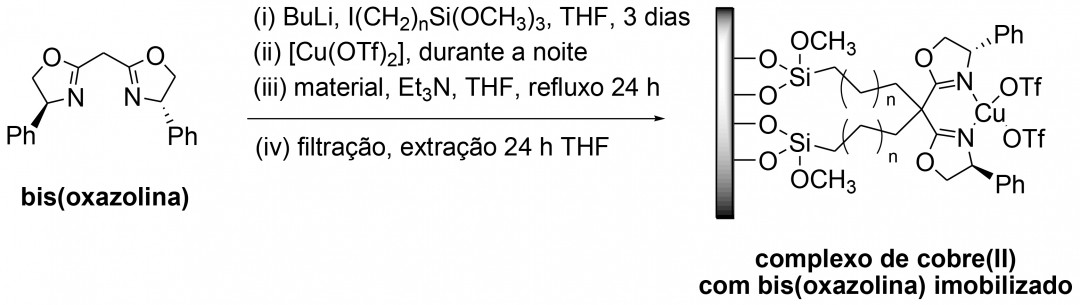
The aim of the present invention is a methodology that allows the effective immobilisation of a high cost commercial bis(oxazoline) onto porous solid supports, acting therefore as an asymmetric heterogeneous catalyst of several liquid phase organic reactions. The copper(II) bis(oxazoline) complex is a known efficient homogeneous catalyst, for example for the kinetic resolution of 1,2-diols, but it can not be easily separated from the reaction media at the end of the reaction and the ligand is high cost. When immobilized in a porous support it can be easily separated by simple filtration at the end of liquid phase reactions and reused in more catalytic cycles of organic reactions, in some cases without significant loss of catalytic activity and enantioselectivity, such as in the case of hydrobenzoin benzoylation.
The present invention is useful for the preparation of asymmetric heterogeneous catalysts for example the kinetic resolution of 1,2-diols in liquid phase.
Innovative aspects & main advantages
The present invention presents the following advantages in relation to the existent technologies: the materials prepared by this methodology are efficient heterogeneous catalysts in the asymmetric benzoylation of 1,2-diols, and eventually in other asymmetric organic transformations, can be easily filtered at the end of the liquid phase reaction and reused in more catalytic cycles without loss of catalytic activity and enantioselectivity.
Applications
Organic compounds synthesis for pharmaceuticals and agrochemicals.